A trisubstituted cyclohexane compound is given – Trisubstituted cyclohexane compounds, characterized by their unique molecular structure, exhibit intriguing stereochemistry and reactivity, making them valuable in various scientific disciplines. This comprehensive overview delves into the structural features, stereochemistry, reactivity, synthesis, and applications of these versatile compounds.
The molecular structure of trisubstituted cyclohexane compounds consists of a cyclohexane ring with three substituents attached to different carbon atoms. The relative positions of these substituents give rise to different types of trisubstituted cyclohexane compounds, each with distinct physical and chemical properties.
Structural Features of a Trisubstituted Cyclohexane Compound
Trisubstituted cyclohexane compounds are organic molecules that contain a cyclohexane ring with three substituents attached to it. The molecular structure of these compounds can be represented as follows:

The substituents can be any type of atom or group of atoms, and they can be located at any of the six carbon atoms in the cyclohexane ring. The relative positions of the substituents determine the different types of trisubstituted cyclohexane compounds.
Types of Trisubstituted Cyclohexane Compounds, A trisubstituted cyclohexane compound is given
- 1,1,1-Trisubstituted cyclohexaneshave all three substituents attached to the same carbon atom.
- 1,1,2-Trisubstituted cyclohexaneshave two substituents attached to one carbon atom and the third substituent attached to an adjacent carbon atom.
- 1,2,2-Trisubstituted cyclohexaneshave two substituents attached to one carbon atom and the third substituent attached to the carbon atom opposite the first two substituents.
- 1,2,3-Trisubstituted cyclohexaneshave one substituent attached to each of three different carbon atoms.
- 1,3,5-Trisubstituted cyclohexaneshave one substituent attached to each of three carbon atoms that are separated by two other carbon atoms.
Stereochemistry of Trisubstituted Cyclohexane Compounds
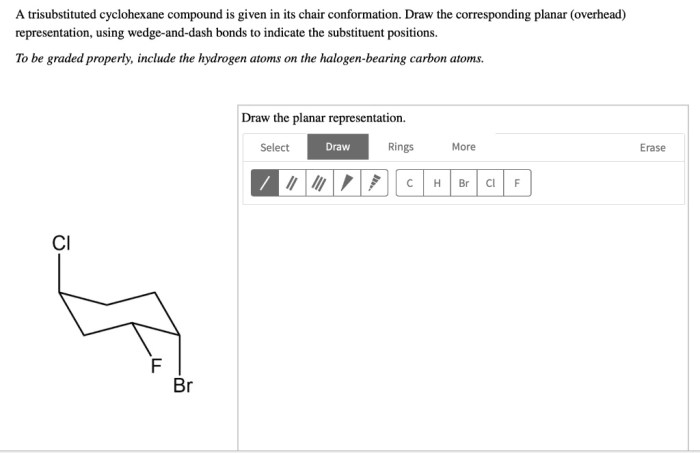
The stereochemistry of trisubstituted cyclohexane compounds refers to the spatial arrangement of the substituents around the cyclohexane ring. Trisubstituted cyclohexane compounds can exist as stereoisomers, which are molecules that have the same molecular formula and connectivity but different spatial arrangements of their atoms.
The different stereoisomers of trisubstituted cyclohexane compounds are determined by the relative orientations of the substituents on the cyclohexane ring. For example, 1,2,3-trisubstituted cyclohexanes can exist as two stereoisomers, which are known as the axial and equatorial isomers.
Axial and Equatorial Isomers
In axial isomers, two of the substituents are located on the same side of the cyclohexane ring, while the third substituent is located on the opposite side of the ring. In equatorial isomers, all three substituents are located on the same side of the ring.

The axial and equatorial isomers of trisubstituted cyclohexane compounds have different physical and chemical properties. For example, axial isomers are generally less stable than equatorial isomers due to steric hindrance between the axial substituents and the hydrogen atoms on the cyclohexane ring.
Reactivity of Trisubstituted Cyclohexane Compounds
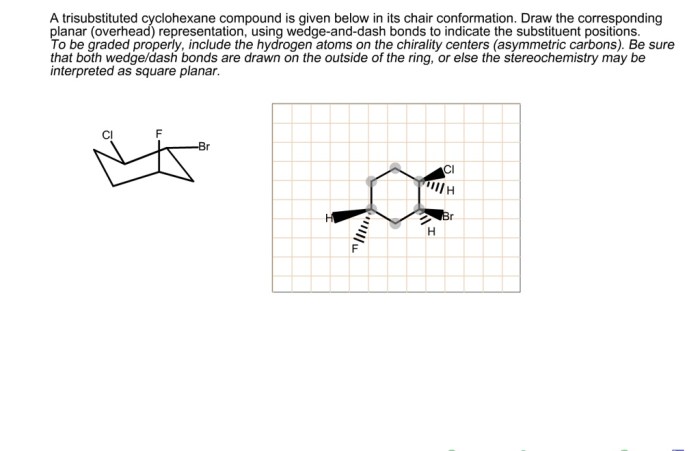
The reactivity of trisubstituted cyclohexane compounds depends on the nature of the substituents and their relative positions on the cyclohexane ring. Substituents that are electron-withdrawing, such as halogens and carbonyl groups, can decrease the reactivity of the cyclohexane ring, while substituents that are electron-donating, such as alkyl groups and alkoxy groups, can increase the reactivity of the ring.
The relative positions of the substituents can also affect the reactivity of the cyclohexane ring. For example, 1,2-disubstituted cyclohexanes are more reactive than 1,3-disubstituted cyclohexanes due to the increased steric hindrance in the 1,3-disubstituted cyclohexanes.
Synthesis of Trisubstituted Cyclohexane Compounds: A Trisubstituted Cyclohexane Compound Is Given
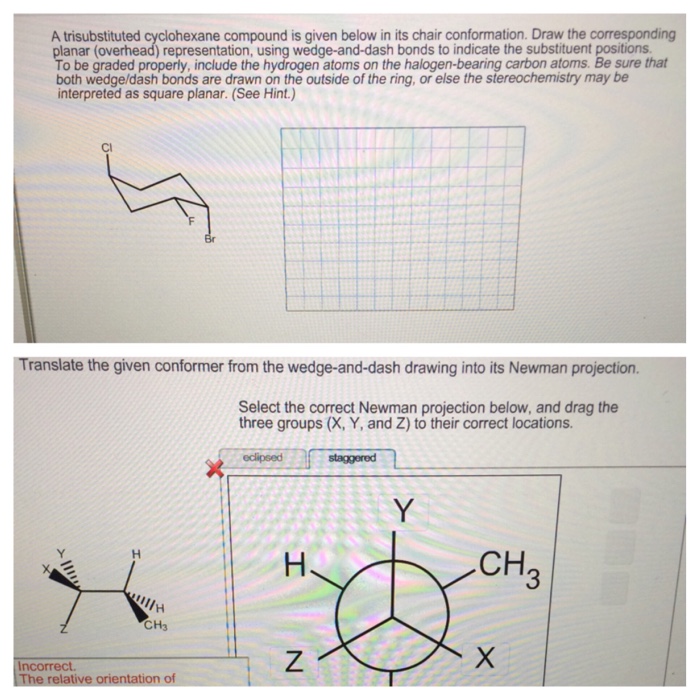
Trisubstituted cyclohexane compounds can be synthesized using a variety of methods. One common method is the Diels-Alder reaction, which involves the cycloaddition of a conjugated diene with a dienophile.
Another common method is the Friedel-Crafts alkylation, which involves the reaction of an aromatic compound with an alkyl halide in the presence of a Lewis acid catalyst.
Applications of Trisubstituted Cyclohexane Compounds
Trisubstituted cyclohexane compounds are used in a variety of applications, including:
- Pharmaceuticals: Trisubstituted cyclohexane compounds are used as the active ingredients in a variety of pharmaceuticals, such as steroids and antibiotics.
- Materials science: Trisubstituted cyclohexane compounds are used as the building blocks for a variety of polymers and plastics.
- Other industries: Trisubstituted cyclohexane compounds are also used in a variety of other industries, such as the food industry, the cosmetics industry, and the automotive industry.
Questions Often Asked
What is the difference between cis and trans trisubstituted cyclohexane compounds?
Cis-trans isomerism occurs when two substituents are attached to the same carbon atom. In cis isomers, the substituents are on the same side of the ring, while in trans isomers, they are on opposite sides.
How can trisubstituted cyclohexane compounds be synthesized?
Trisubstituted cyclohexane compounds can be synthesized through various methods, including Diels-Alder reactions, Friedel-Crafts alkylation, and catalytic hydrogenation.
What are the applications of trisubstituted cyclohexane compounds?
Trisubstituted cyclohexane compounds have applications in pharmaceuticals, materials science, and chemical research. They are used as intermediates in the synthesis of complex organic molecules, as chiral auxiliaries in asymmetric synthesis, and as building blocks in the construction of supramolecular structures.