Elements compounds and mixtures worksheet with answers pdf – Delve into the realm of chemistry with our comprehensive Elements, Compounds, and Mixtures Worksheet with Answers PDF. This meticulously crafted resource empowers you to grasp the fundamental concepts of these substances and their interactions, providing a solid foundation for further exploration in the field.
Our worksheet is designed to engage and educate, offering a structured approach to understanding the properties, identification, separation, and applications of elements, compounds, and mixtures. Dive into the fascinating world of chemical reactions and discover the practical significance of these substances in various industries.
Elements, Compounds, and Mixtures Basics
Elements, compounds, and mixtures are the fundamental building blocks of matter. Understanding their properties and behavior is essential in chemistry.
An element is a pure substance that cannot be broken down into simpler substances by chemical means. Examples of elements include oxygen, hydrogen, and gold.
A compound is a pure substance that is composed of two or more elements chemically combined in fixed proportions. Examples of compounds include water (H 2O), carbon dioxide (CO 2), and salt (NaCl).
A mixture is a combination of two or more elements or compounds that are not chemically combined. The composition of a mixture can vary. Examples of mixtures include saltwater, air, and alloys.
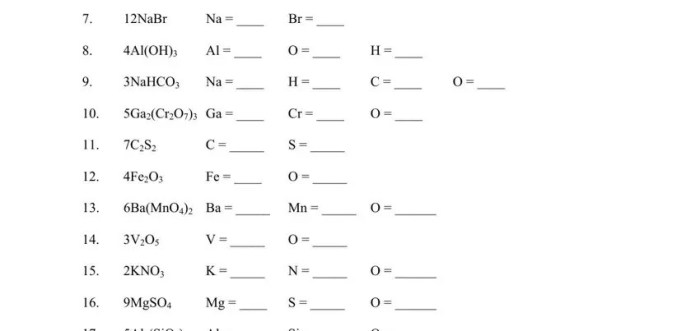
Identifying Elements, Compounds, and Mixtures
Identifying elements, compounds, and mixtures can be done through various methods:
- Physical properties:Elements, compounds, and mixtures have distinct physical properties such as color, density, and melting point. These properties can help in their identification.
- Chemical properties:Elements, compounds, and mixtures undergo different chemical reactions. Identifying the products of these reactions can aid in their classification.
- Spectroscopic methods:Spectroscopic techniques, such as mass spectrometry and infrared spectroscopy, can provide information about the composition and structure of elements, compounds, and mixtures.
Separating Elements, Compounds, and Mixtures
Separating elements, compounds, and mixtures is essential for various purposes. Techniques used for separation include:
- Filtration:Filtration is used to separate solids from liquids or gases. A filter paper or membrane allows the liquid or gas to pass through while retaining the solids.
- Distillation:Distillation is used to separate liquids based on their boiling points. The liquid mixture is heated, and the vapors are condensed to collect the desired component.
- Chromatography:Chromatography is a technique used to separate compounds based on their different affinities for a stationary and mobile phase. The mixture is passed through a stationary phase, and the components are separated as they move through the mobile phase.
Chemical Reactions Involving Elements, Compounds, and Mixtures, Elements compounds and mixtures worksheet with answers pdf
Elements, compounds, and mixtures can undergo various chemical reactions. These reactions can be classified into different types:
- Combination reactions:Two or more substances combine to form a single product.
- Decomposition reactions:A single substance breaks down into two or more products.
- Single-replacement reactions:One element replaces another element in a compound.
- Double-replacement reactions:Two compounds exchange ions to form two new compounds.
Applications of Elements, Compounds, and Mixtures
Elements, compounds, and mixtures have numerous applications in everyday life and various industries:
- Medicine:Elements like iodine and compounds like aspirin are used in medications.
- Technology:Elements like silicon and compounds like semiconductors are used in electronic devices.
- Agriculture:Compounds like fertilizers and pesticides are used to enhance crop production.
Essential Questionnaire: Elements Compounds And Mixtures Worksheet With Answers Pdf
What is the difference between an element, compound, and mixture?
An element is a pure substance that cannot be broken down into simpler substances. A compound is a substance composed of two or more elements chemically combined. A mixture is a combination of two or more substances that are not chemically combined.
How can I identify an element, compound, or mixture?
Elements can be identified by their unique properties, such as their atomic number and atomic mass. Compounds can be identified by their chemical formula, which shows the elements that make them up and their proportions. Mixtures can be identified by their physical properties, such as their color, density, and solubility.
How can I separate an element, compound, or mixture?
Elements, compounds, and mixtures can be separated using a variety of techniques, such as distillation, filtration, and chromatography. The specific technique used depends on the properties of the substances being separated.