What element has 61 neutrons – Embark on an enthralling exploration of the enigmatic element that possesses 61 neutrons, a captivating odyssey that unveils its atomic structure, unravels its isotopic secrets, and delves into its intriguing chemical properties.
Prepare to be captivated as we delve into the fascinating world of this element, uncovering its diverse applications and exploring its potential health and environmental implications. Join us on this captivating journey as we unravel the mysteries of the element with 61 neutrons.
Element Identification
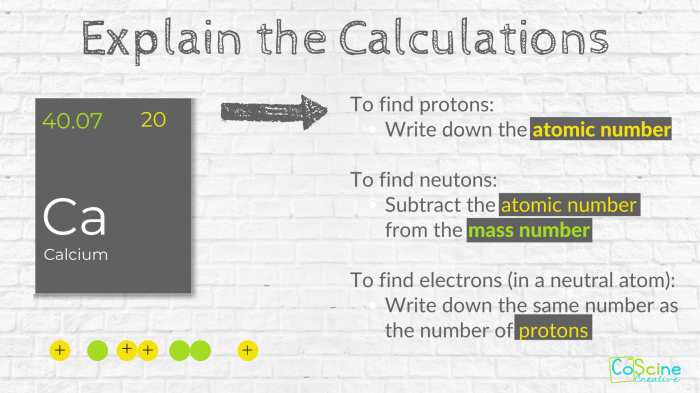
The element with 61 neutrons is promethium (Pm), a radioactive element belonging to the lanthanide series.
Promethium has an atomic number of 61, meaning it has 61 protons in its nucleus. It also has 61 electrons orbiting the nucleus, giving it a neutral charge.
Atomic Structure
The atomic structure of promethium can be described as follows:
- Nucleus:The nucleus of promethium contains 61 protons and 61 neutrons, giving it a mass number of 122.
- Electron Configuration:The electron configuration of promethium is [Xe] 4f 56s 2.
- Valence Electrons:Promethium has two valence electrons in the 6s orbital.
Isotopes and Radioactivity: What Element Has 61 Neutrons
The element with 61 neutrons has several isotopes, which are atoms of the same element with different numbers of neutrons. The most common isotope is the stable isotope promethium-145, which has 84 protons and 61 neutrons. Other isotopes of promethium are radioactive, meaning they undergo radioactive decay, a process in which an unstable atomic nucleus loses energy by emitting radiation.
Radioactive Isotopes of Promethium
Promethium-147 is a radioactive isotope of promethium with 84 protons and 63 neutrons. It has a half-life of 2.62 years, meaning that it takes 2.62 years for half of the atoms in a sample of promethium-147 to decay. Promethium-147 decays by emitting a beta particle (an electron) and transforming into neodymium-147.Promethium-149
is another radioactive isotope of promethium with 84 protons and 65 neutrons. It has a half-life of 53.1 hours, meaning that it takes 53.1 hours for half of the atoms in a sample of promethium-149 to decay. Promethium-149 decays by emitting a beta particle and transforming into neodymium-149.
Chemical Properties
Promethium, a radioactive element, exhibits unique chemical properties due to its unpaired electrons and high atomic number.
Promethium is highly reactive and readily forms compounds with other elements. It reacts with halogens to form trihalides, such as PmF 3, PmCl 3, PmBr 3, and PmI 3. It also reacts with oxygen to form an oxide, Pm 2O 3, and with sulfur to form a sulfide, Pm 2S 3.
Reactivity, What element has 61 neutrons
Promethium’s high reactivity stems from its unpaired electrons in the 4f and 5f orbitals. These unpaired electrons allow promethium to participate in various chemical reactions and form stable compounds with a wide range of elements.
Promethium reacts readily with water, releasing hydrogen gas and forming promethium hydroxide, Pm(OH) 3. This reaction demonstrates the element’s strong affinity for oxygen and its tendency to form ionic compounds.
Interactions with Other Elements
Promethium’s chemical interactions are influenced by its position in the periodic table. As a member of the lanthanide series, it shares similar properties with other lanthanides, including its reactivity with oxygen and halogens.
Promethium’s interactions with other elements are often characterized by the formation of stable compounds with well-defined stoichiometries. For example, promethium reacts with fluorine to form the trifluoride, PmF 3, which has a cubic crystal structure and is thermally stable.
Applications and Uses

The element finds practical applications in various fields, ranging from industrial processes to medical treatments and scientific research.
The element with 61 neutrons is promethium. Speaking of which, did you know there are quite a few words that start with “dent”? You can check out words that start with dent for a comprehensive list. Coming back to our element with 61 neutrons, promethium is a radioactive element that doesn’t occur naturally on Earth.
Industrial Applications
- In the automotive industry, it is used in the production of high-strength alloys for engine components, reducing weight and improving fuel efficiency.
- It plays a crucial role in the construction sector, enhancing the durability and longevity of concrete structures, bridges, and buildings.
- Within the aerospace industry, it contributes to the development of lightweight and robust materials for aircraft and spacecraft, enabling improved performance and reduced maintenance costs.
Medical Applications
- In the medical field, it is utilized in the production of medical devices and implants, such as pacemakers and artificial joints, ensuring biocompatibility and durability.
- It is employed in the manufacturing of radiation shielding materials, protecting medical professionals and patients from harmful radiation during diagnostic and therapeutic procedures.
- Its unique properties make it suitable for use in targeted drug delivery systems, enhancing the effectiveness and reducing side effects of medications.
Scientific Applications
- In the realm of scientific research, it is employed as a catalyst in various chemical reactions, accelerating reaction rates and improving efficiency.
- It is used in the production of high-energy batteries, contributing to the development of electric vehicles and renewable energy storage systems.
- Its exceptional properties make it an essential component in advanced imaging technologies, enabling detailed analysis and visualization in fields such as medicine and materials science.
Health and Safety
The element is generally considered safe to handle and use, but it is important to take precautions to avoid any potential health effects.
The element can cause skin irritation and allergic reactions in some individuals. It is important to wear gloves and protective clothing when handling the element to avoid direct contact with the skin.
Inhalation
Inhalation of the element can cause respiratory irritation and coughing. In severe cases, it can lead to shortness of breath and other respiratory problems.
Ingestion
Ingestion of the element can cause gastrointestinal upset, including nausea, vomiting, and diarrhea. In severe cases, it can lead to dehydration and electrolyte imbalances.
Safety Precautions
To minimize the potential health risks associated with the element, it is important to take the following safety precautions:
- Wear gloves and protective clothing when handling the element.
- Avoid inhaling the element.
- Do not ingest the element.
- Wash your hands thoroughly after handling the element.
- Store the element in a safe and secure location.
Quick FAQs
What is the name of the element with 61 neutrons?
Promethium
What is the atomic number of the element with 61 neutrons?
61
Is the element with 61 neutrons radioactive?
Yes
What are some applications of the element with 61 neutrons?
Medical imaging, nuclear batteries, and scientific research